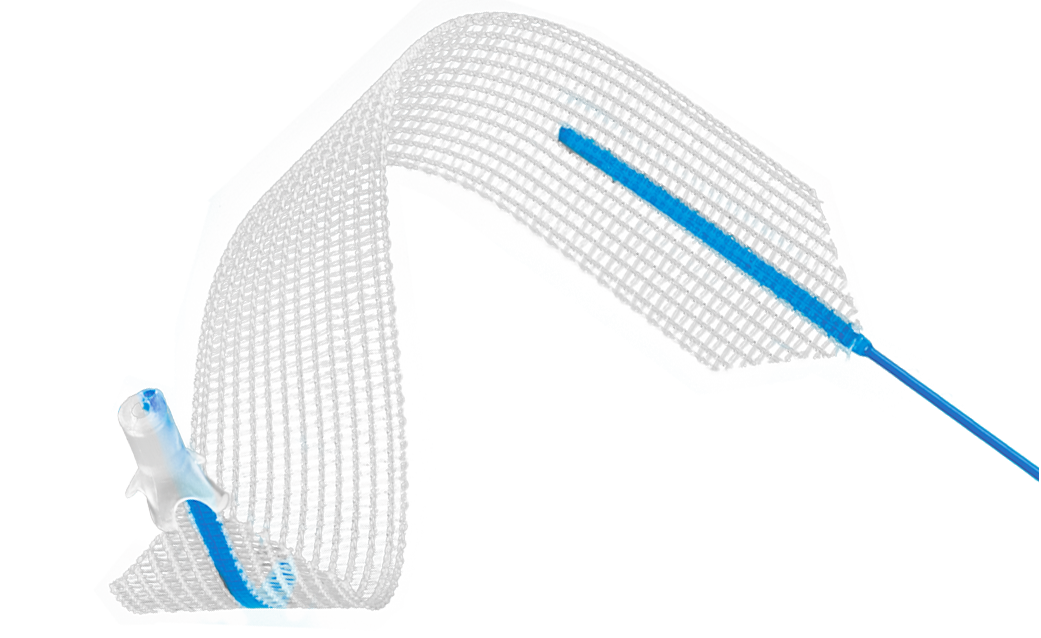
Altis® Brief Statement
Indications
The Altis® Single Incision Sling System is indicated for the treatment of female stress urinary incontinence (SUI) resulting from urethral hypermobility and/or intrinsic sphincter deficiency (ISD).
Contraindications
It is the responsibility of the physician to advise the prospective patients or their representatives, prior to surgery, of the contraindications associated with the use of this product. The Altis Single Incision Sling System is contraindicated for use in patients with the following conditions:
- Pregnancy or desire for future pregnancy
- Potential for further growth (e.g., adolescents)
- Known active urinary tract infection and/or infection in operative field
- Taking anti-coagulant therapy
- Abnormal urethra (e.g., fistula, diverticulum)
- Intraoperative urethral injury
- Any condition, including known or suspected pelvic pathology, which could compromise implant or implant placement
- Sensitivity/allergy to polypropylene
Warnings and Precautions
It is the responsibility of the physician to advise the prospective patients or their representatives, prior to surgery, of the warnings and precautions associated with the use of this product and the associated surgical risks.
Warnings
The Altis Single Incision Sling System should only be used by physicians familiar with the surgical procedures and techniques involving transvaginal placement of non-absorbable, synthetic mesh slings and who have adequate education and experience in the treatment of female SUI.
A thorough assessment of each patient should be made to determine the suitability of a synthetic mesh sling procedure.
The patient should be counseled that alternative incontinence treatments may be appropriate, and the reason for choosing a mesh sling procedure should be explained.
Obtain patient consent prior to surgery and ensure that the patient has an understanding of the postoperative risks and potential complications of transvaginal mesh sling surgery.
Patient counseling should include a discussion that the sling to be implanted is a permanent implant and that some complications associated with the implanted mesh sling may require additional surgery; repeat surgery may not resolve these complications. Serious adverse tissue responses or infection may require removal of mesh, and complete removal of the sling may not always be possible. Individuals may have varying degrees of collagen laydown that may result in scarring.
As with all surgical procedures, patients with certain underlying conditions may be more susceptible to postoperative bleeding, impaired blood supply, compromised/delayed healing, or other complications and adverse events.
The risks and benefits of using Altis should be considered in patients.
Any future pregnancy could negate the benefits of this surgical procedure. Patients should report any bleeding, pain, abnormal vaginal discharge or sign of infection that occur at any time.
The procedure to insert the Altis sling requires good knowledge of pelvic anatomy and the correct use of the introducer needles in order to avoid damage to adjacent anatomical structures.
Cystoscopy should be performed to confirm bladder and urethral integrity.
Avoid placing excessive tension on the Altis sling during placement and adjustment to maintain sling integrity and to avoid compression of the urethra when tensioning.
Potential Complications
Potential complications include mesh extrusion, pelvic/urogenital pain, groin pain, hip pain (may be related to patient positioning), urinary retention, bleeding, de novo urgency, delayed wound healing, dyspareunia, hip/groin pain, inflammation, nausea, overactive bladder, pain, pelvic hematoma, reaction to antibiotic, slight discomfort upon return to work, urinary tract infection, urine stream decreased, and voiding dysfunction.
Adverse events are known to occur with transvaginal synthetic sling procedures and implants. Adverse events following mesh implantation may be de novo, persistent, worsening, transient, or permanent.
Additional potential complications include, but are not limited to, abscess (acute or delayed), adhesion/scar formation, allergy, hypersensitivity or other immune reaction, bleeding, hemorrhage or hematoma, dehiscence, delayed wound healing, extrusion, erosion or exposure of mesh sling into the vagina or other structures or organs, fistula formation, infection, inflammation (acute or chronic), local irritation, necrosis, de novo and/or worsening dyspareunia, neuromuscular symptoms (acute or chronic), partner pain and/or discomfort during intercourse, perforation or injury of soft tissue (e.g., muscles, nerves, vessels), structures, or organs (e.g., bone, bladder, urethra, ureters, vagina), seroma, sling migration, suture erosion, bladder storage dysfunction (e.g., increased daytime frequency, urgency, nocturia, overactive bladder, urinary incontinence), ureteral obstruction, urinary tract infection, voiding symptoms (e.g., dysuria, urinary retention, incomplete emptying, straining, positional voiding, weak stream), granulation tissue formation, palpable mesh (patient and/or partner), sexual dysfunction, vaginal discharge (abnormal) and vaginal scarring or tightening.
The occurrence of these events may require one or more revision surgeries, including removal of the sling.
Complete removal of the sling may not always be possible, and additional surgeries may not always fully correct the complications.
There may be unresolved pain with or without mesh sling explanation.
The information provided is not comprehensive with regard to product risks. For a comprehensive listing of indications, contraindications, warnings, precautions, and adverse events refer to the product’s Instructions for Use. Alternatively, you may contact a Coloplast representative at 1-800-258-3476 and/or visit the company Website at www.coloplast.com.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
Minneapolis, MN
01/19/2021
PM-03363
Restorelle® Y Polypropylene Mesh for Sacrocolposuspension/Sacrocolpopexy Brief Statement
Indications
Restorelle Y is indicated for use as a bridging material for sacrocolposuspension/sacrocolpopexy (transabdominal placement via laparotomy, laparoscopic, or robotic approach) where surgical treatment for vaginal vault prolapse is warranted.
Contraindications
It is the responsibility of the physician to advise the prospective patients or their representatives, prior to surgery, of the contraindications associated with the use of this product.
The Restorelle Y is contraindicated for use in patients with the following conditions:
- Pregnancy or desire for future pregnancy
- Potential for further growth (e.g., adolescents)
- Pre-existing local or systemic infection. Treat the infection with the appropriate antiseptics and/or antibiotics to eliminate the infection before placing the Restorelle Y mesh.
- Taking anti-coagulant therapy
- Any condition, including known or suspected pelvic pathology, which could compromise implant or implant placement
- Sensitivity/allergy to polypropylene
Warnings and Precautions
It is the responsibility of the physician to advise the prospective patients or their representatives, prior to surgery, of the warnings and precautions associated with the use of this product and the associated surgical risks.
Warnings
Restorelle Y mesh should only be used by physicians familiar with the surgical procedures and techniques involving non-absorbable mesh and who have adequate education and experience in the treatment of pelvic organ prolapse.
A thorough assessment of each patient should be made to determine the suitability of a synthetic mesh procedure.
The patient should be counseled that alternative incontinence treatments may be appropriate, and the reason for choosing a mesh procedure should be explained.
Obtain patient consent prior to surgery and ensure that the patient has an understanding of the postoperative risks and potential complications of transabdominal mesh surgery.
Patient counseling should include a discussion that the mesh to be implanted is a permanent implant and that some complications associated with the implanted mesh may require additional surgery; repeat surgery may not resolve these complications. Serious adverse tissue responses or infection may require removal of mesh, and complete removal of the mesh may not always be possible. Individuals may have varying degrees of collagen laydown that may result in scarring.
As with all surgical procedures, patients with certain underlying conditions may be more susceptible to postoperative bleeding, impaired blood supply, compromised/delayed healing, or other complications and adverse events.
The risks and benefits of using Restorelle Y should be considered in patients.
Any future pregnancy could negate the benefits of this surgical procedure. Patients should report any bleeding, pain, abnormal vaginal discharge or sign of infection that occur at any time.
Do not use product that has damaged or opened packaging, or has expired, as sterility may be compromised.
The procedure to insert the Restorelle Y mesh requires good knowledge of pelvic anatomy.
Cystoscopy should be performed to confirm bladder and urethral integrity.
A digital rectal exam should be performed to detect possible rectal perforation.
Avoid placing excessive tension on the Restorelle Y mesh implant during placement and adjustment to maintain mesh integrity.
There should be an appropriate margin of mesh extending beyond the fixation points.
Inadequate fixation of the mesh material to the pelvic tissue may lead to failure of the repair and recurrence of the prolapse.
Precautions
Restorelle Y mesh is provided sterile (ethylene oxide sterilization) and is for single-use only.
Use caution to prevent intraoperative injury to adjacent pelvic structures.
Do not let the Restorelle Y come into contact with sharp objects (e.g., staples, clips, or clamps) which could cause damage to the mesh.
Potential Complications
Adverse events are known to occur with transabdominal synthetic mesh procedures and implants. Adverse events following mesh implantation may be de novo, persistent, worsening, transient, or permanent.
Potential complications include, but are not limited to, abscess (acute or delayed), adhesion/scar formation, allergy, hypersensitivity or other immune reaction, bleeding, hemorrhage or hematoma, bowel obstruction, constipation and/or defecatory dysfunction, fecal incontinence and/or anal sphincter incompetence, ileus, dehiscence, delayed wound healing, extrusion, erosion or exposure of mesh into the vagina or other structures or organs, fistula formation, infection, inflammation (acute or chronic), local irritation, mesh migration, necrosis, de novo and/or worsening dyspareunia, neuromuscular symptoms (acute or chronic), pain (acute or chronic), partner pain and/or discomfort during intercourse, perforation or injury of soft tissue (e.g., ligaments, muscles, nerves, vessels), structures, or organs (e.g., bowel, rectum, bladder, urethra, ureters, vagina), seroma, suture erosion, bladder storage dysfunction (e.g., increased daytime frequency, urgency, nocturia, overactive bladder, urinary incontinence), ureteral obstruction, urinary tract infection, voiding symptoms (e.g., dysuria, urinary retention, incomplete emptying, straining, positional voiding, weak stream), de novo or worsening prolapse in untreated compartment, granulation tissue formation, palpable mesh (patient and/or partner), recurrent prolapse, sexual dysfunction, vaginal discharge (abnormal) and vaginal scarring, tightening, rigidity, shortening and/or contracture.
The occurrence of adverse events may require one or more revision surgeries, including removal of the mesh.
Complete removal of the mesh may not always be possible, and additional surgeries may not always fully correct the complications.
There may be unresolved pain with or without mesh explantation.
The information provided is not comprehensive with regard to product risks. For a comprehensive listing of indications, contraindications, warnings, precautions, and adverse events refer to the product’s Instructions for Use. Alternatively, you may contact a Coloplast representative at 1-800-258-3476 and/or visit the company Website at www.coloplast.com.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
Minneapolis, MN
02/22/2018
PM-03856
Restorelle® M, L and XL Polypropylene Mesh for Sacrocolposuspension/Sacrocolpopexy Brief Statement
Indications
Restorelle M, Restorelle L and Restorelle XL are indicated for use as a bridging material for sacrocolposuspension/sacrocolpopexy (transabdominal placement via laparotomy, laparoscopic, or robotic approach) where surgical treatment for vaginal vault prolapse is warranted.
Contraindications
It is the responsibility of the physician to advise the prospective patients or their representatives, prior to surgery, of the contraindications associated with the use of this product.
The Restorelle M, L and XL are contraindicated for use in patients with the following conditions:
- Pregnancy or desire for future pregnancy
- Potential for further growth (e.g., adolescents)
- Pre-existing local or systemic infection. Treat the infection with the appropriate antiseptics and/or antibiotics to eliminate the infection before placing the Restorelle M, L or XL mesh.
- Taking anti-coagulant therapy
- Any condition, including known or suspected pelvic pathology, which could compromise implant or implant placement
- Sensitivity/allergy to polypropylene
Warnings and Precautions
It is the responsibility of the physician to advise the prospective patients or their representatives, prior to surgery, of the warnings and precautions associated with the use of this product and the associated surgical risks.
Warnings
The Restorelle M, L and XL mesh should only be used by physicians familiar with the surgical procedures and techniques involving non-absorbable mesh and who have adequate education and experience in the treatment of pelvic organ prolapse.
A thorough assessment of each patient should be made to determine the suitability of a synthetic mesh procedure.
The patient should be counseled that alternative incontinence treatments may be appropriate, and the reason for choosing a mesh procedure should be explained.
Obtain patient consent prior to surgery and ensure that the patient has an understanding of the postoperative risks and potential complications of transabdominal mesh surgery.
Patient counseling should include a discussion that the mesh to be implanted is a permanent implant and that some complications associated with the implanted mesh may require additional surgery; repeat surgery may not resolve these complications. Serious adverse tissue responses or infection may require removal of mesh, and complete removal of the mesh may not always be possible. Individuals may have varying degrees of collagen laydown that may result in scarring.
As with all surgical procedures, patients with certain underlying conditions may be more susceptible to postoperative bleeding, impaired blood supply, compromised/delayed healing, or other complications and adverse events.
The risks and benefits of using Restorelle M, L or XL should be considered in patients.
Any future pregnancy could negate the benefits of this surgical procedure. Patients should report any bleeding, pain, abnormal vaginal discharge or sign of infection that occur at any time.
Do not use product that has damaged or opened packaging, or has expired, as sterility may be compromised.
The procedure to insert the Restorelle M, L or XL requires good knowledge of pelvic anatomy.
Cystoscopy should be performed to confirm bladder and urethral integrity.
A digital rectal exam should be performed to detect possible rectal perforation.
Avoid placing excessive tension on the Restorelle M, L or XL mesh implant during placement and adjustment to maintain mesh integrity.
There should be an appropriate margin of mesh extending beyond the fixation points.
Inadequate fixation of the mesh material to the pelvic tissue may lead to failure of the repair and recurrence of the prolapse.
Precautions
Restorelle M, L and XL mesh is provided sterile (ethylene oxide sterilization) and is for single-use only.
Use caution to prevent intraoperative injury to adjacent pelvic structures.
Do not let the Restorelle M, L or XL come into contact with sharp objects (e.g., staples, clips, or clamps) which could cause damage to the mesh.
Potential Complications
Adverse events are known to occur with transabdominal synthetic mesh procedures and implants. Adverse events following mesh implantation may be de novo, persistent, worsening, transient, or permanent.
Potential complications include, but are not limited to, abscess (acute or delayed), adhesion/scar formation, allergy, hypersensitivity or other immune reaction, bleeding, hemorrhage or hematoma, bowel obstruction, constipation and/or defecatory dysfunction, fecal incontinence and/or anal sphincter incompetence, ileus, dehiscence, delayed wound healing, extrusion, erosion or exposure of mesh into the vagina or other structures or organs, fistula formation, infection, inflammation (acute or chronic), local irritation, mesh migration, necrosis, de novo and/or worsening dyspareunia, neuromuscular symptoms (acute or chronic), pain (acute or chronic), partner pain and/or discomfort during intercourse, perforation or injury of soft tissue (e.g., ligaments, muscles, nerves, vessels), structures, or organs (e.g., bowel, rectum, bladder, urethra, ureters, vagina), seroma, suture erosion, bladder storage dysfunction (e.g., increased daytime frequency, urgency, nocturia, overactive bladder, urinary incontinence), ureteral obstruction, urinary tract infection, voiding symptoms (e.g., dysuria, urinary retention, incomplete emptying, straining, positional voiding, weak stream), de novo or worsening prolapse in untreated compartment, granulation tissue formation, palpable mesh (patient and/or partner), recurrent prolapse, sexual dysfunction, vaginal discharge (abnormal) and vaginal scarring, tightening, rigidity, shortening and/or contracture.
The occurrence of adverse events may require one or more revision surgeries, including removal of the mesh.
Complete removal of the mesh may not always be possible, and additional surgeries may not always fully correct the complications.
There may be unresolved pain with or without mesh explantation.
The information provided is not comprehensive with regard to product risks. For a comprehensive listing of indications, contraindications, warnings, precautions, and adverse events refer to the product’s Instructions for Use. Alternatively, you may contact a Coloplast representative at 1-800-258-3476 and/or visit the company Website at www.coloplast.com.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
Minneapolis, MN
02/22/2018
PM-03862