Medical procedures and environmental hazards
For the Intibia™ Implantable Tibial Nerve Stimulator
![]()
Magnetic Resonance (MR) Conditional: A patient with the INTIBIA ITNS may be safely scanned under the following conditions. Failure to follow these conditions may results in injury to the patient.
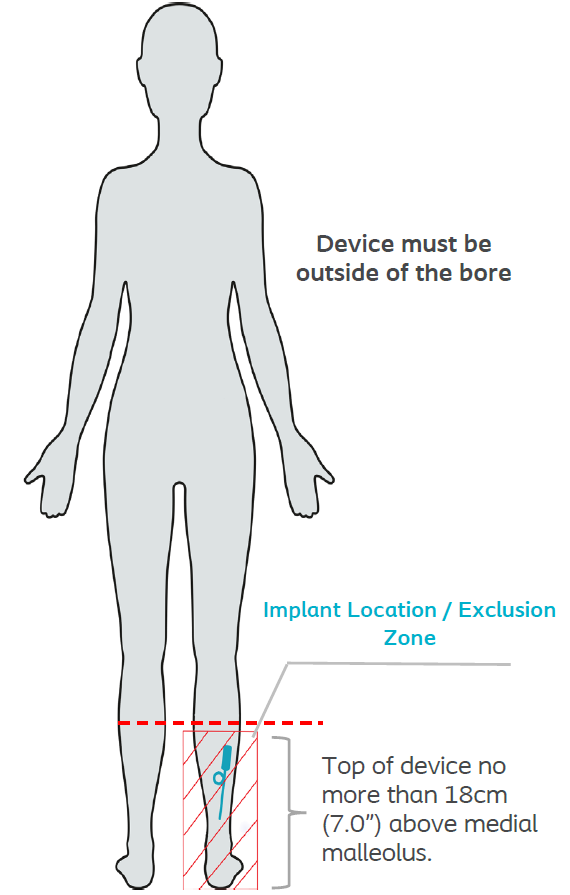
Use of any Local Transmit / Receive Coil is permissible if placed outside of the implant location / Exclusion Zone.
| Name/Identification of device | INTIBIA ITNS |
| Nominal value(s) of Static Magnetic Field [T] | 1.5 T or 3 T |
| Maximum Spatial Field Gradient [T/m and gauss/cm] | 20 T/m (2000 gauss/cm) |
| RF Excitation | Circularly Polarized (CP) |
| RF Transmit Coil Type | Whole body transmit coil. Any local Transmit/Receive coil may be used, provided it is not over the implant location / Exclusion Zone. |
| 1.5 T and 3 T SAR Levels | MR imaging is only safe when the device is outside the bore. Under these conditions the maximum SAR limit is Normal Mode (2 W/kg Whole Body average or 3.2 W/kg Head Average SAR). |
| Limits on Scan Duration | 15 minutes of continuous RF (a sequence or back to back series/scan without breaks) | Maximum Gradient Slew Rate (dB/dt) | 200 T/m/s |
| MR Image Artifact | The presence of this implant may produce an image artifact of 38 mm. |
| If information about a specific parameter is not included, there are no conditions associated with that parameter. | |
Ultrasound: The INTIBIA ITNS should not be exposed to therapeutic levels of ultrasound energy, as the implantable part of the active implantable medical device can inadvertently concentrate the ultrasound field and cause harm.
Radiation: Electronic components can be damaged by therapeutic ionizing radiation, and any damage may not be immediately detectable.
Environmental hazards: The INTIBIA ITNS complies with ISO 14708-3:2017 Clause 27 and IEC 60601-1-2 Table 11 for protection from electromagnetic interference (EMI). A strong magnet aligned in proximity to an INTIBIA ITNS will stop therapy. Timed applications of a strong magnet can program an INTIBIA ITNS when its programmer is unavailable. Patients should be advised to seek medical guidance if any unusual sensation occurs at the implant site during exposure to an EMI source.
Other active implantable medical devices: The INTIBIA ITNS has not been tested for interference with other electronic implants such as pacemakers, defibrillators, and neurostimulators. If the INTIBIA ITNS is to be used with other electronic implants, an interaction study must be performed by the physician.
*Caution: Investigational device. Limited by Federal (or United States) law to investigational use.
*This medical device is currently not for sale in any country.